Pérez-Bitrián Lab
Organometallic and Fluorine Chemistry
Humboldt-Universität zu Berlin
Research
We are a young research group at the Institut für Chemie of the Humboldt-Universität zu Berlin (Germany). Our research projects are located in the field of preparative inorganic and organometallic chemistry, at the interface of main group and transition metal chemistry, always with a special component of fluorine chemistry.
The synthesis of the first Xe compound by Bartlett in 1962 was a groundbreaking discovery that changed the paradigm of the inertness of noble gases. A key breakthrough in the history of noble gas compounds was the synthesis of the first compounds containing Xe–C bonds in 1989, [XeC6F5]+ salts. Since then, the field of organoxenon chemistry experienced a great development, making many different species available, which require the use of fluorinated ligands in most of the cases. Although their properties have been thoroughly investigated, their application in synthesis has been barely exploited.
The overall objective of the Pérez-Bitrián Lab is the investigation of the applicability of known and new organoxenon compounds as strong oxidizers for the synthesis of organometallic complexes in high oxidation states. This allows the coordination of the organic moiety to the metal center, which is oxidized at the same time. In this regard, our main target is the study of 3d metals, especially cobalt and nickel, not only because they are of increasing interest in many catalytic processes, but also because the stabilization of their higher oxidation states is more challenging than in their heavier homologues and therefore they have been far less studied. Additionally, such complexes are also relevant as intermediates in catalysis, which enables the investigation of their role in different cross-coupling processes. With this in mind, the incorporation of different fluorinated ligands will require the synthesis of new classes of organoxenon compounds and the study of their properties, which becomes our second main goal.
The use of Schlenk techniques is normally enough in our group, yet some of the xenon compounds and their precursors are extremely reactive and cannot be handled in glassware, but require specific self-built reactors consisting of PFA (perfluoroalkoxy alkanes). Standard spectroscopic and spectrometric techniques are used in the characterization of the compounds, such as multinuclear variable-temperature NMR spectroscopy, IR and Raman spectroscopy, EPR spectroscopy, X-ray diffraction, mass spectrometry, cyclic voltammetry or magnetic measurements. Additionally, quantum-chemical methods will accompany the experimental research. Students have the opportunity to acquire experience in different synthetic methodologies, as well as in a handful of characterization techniques.
Publications
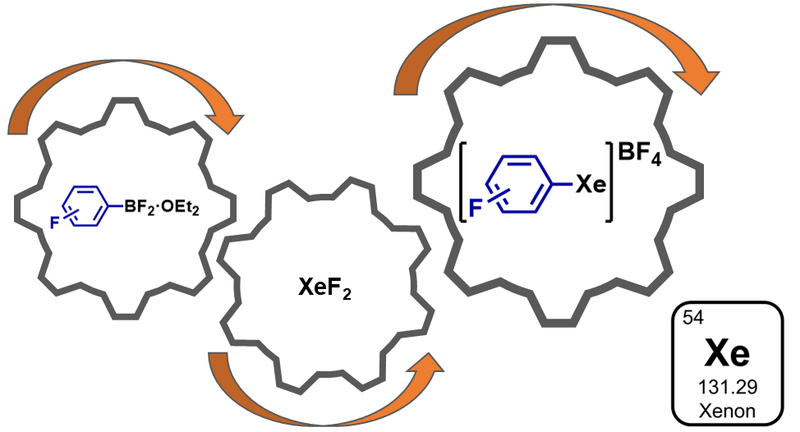
29. A General Approach for the Synthesis of Arylxenonium(II) Tetrafluoroborates
P. Cortés Soláns, M. L. Bubenik, M. H. Lee, P. S. Riemann, A. Pérez-Bitrián*
Inorg. Chem. 2025, 64, 23184−23191.
Work featured in the journal cover.
A. Pérez-Bitrián,* C. Munarriz, A. J. Rueda, A. Martín, J. M. Casas*
Z. Anorg. Allg. Chem. 2026, 652, e202500150.
27. On Pentafluoroorthotellurates and Related Compounds
J. Bader, L. Fischer, K. F. Hoffmann, N. Limberg, A. Millanvois, F. Oesten, A. Pérez-Bitrián, J. Schlögl, A. N. Toraman, D. Wegener, A. Wiesner, S. Riedel*
Chem. Rev. 2025, 125, 9140–9186.
D. Wegener, N. Limberg, M. Bubenik, A. Pérez-Bitrián, A. Wiesner, S. Riedel*
JACS Au 2025, 5, 3565–3574.
25. 20. Deutscher Fluortag in Schmitten
A. Pérez-Bitrián, Nachrichten aus der Chemie 2025, 73, 77.
24. Pentafluoroorthotellurate Uncovered: Theoretical Perspectives on an Extremely Electronegative Group
D. Barrena-Espés, Á. Martín Pendás, S. Riedel, A. Pérez-Bitrián,* J. Munárriz*
Inorg. Chem. 2025, 64, 1064–1074.
23. Gold Trifluoromethyl Complexes as Efficient Regioselective Catalysts in Alkyne Hydration
D. Ruiz-Almoguera, D. Ventura-Espinosa, A. Pérez-Bitrián, A. Martín, J. A. Mata,* M. Baya*
Chem. Eur. J. 2024, 30, e202401753.
22. A Highly Sterically Encumbered Boron Lewis Acid Enabled by an Organotellurium-based Ligand
D. Wegener, A. Pérez-Bitrián, N. Limberg, A. Wiesner, K. F. Hoffmann, S. Riedel*
Chem. Eur. J. 2024, 30, e202401231.
21. Silver(I) Perfluoroalcoholates: Synthesis, Structure, and their Use as Transfer Reagents
P. Golz, K. Shakeri, L. Maas, M. Balizs, A. Pérez-Bitrián, H. D. Kemmler, M. Kleoff, P. Voßnacker, M. Christmann, S. Riedel*
Chem. Eur. J. 2024, 30, e202400861.
20. Questing for Homoleptic Mononuclear Manganese Complexes with Monodentate O-Donor Ligands
A. Pérez-Bitrián,* J. Munárriz,* K. B. Krause, J. Schlögl, K. F. Hoffmann, J. S. Sturm, A. N. Hadi, C. Teutloff, A. Wiesner, C. Limberg, S. Riedel*
Chem. Sci. 2024, 15, 5564–5572.
19. [Xe(OTeF5)(pyF)]+: A Strong Oxidizing Xenonium(II) Teflate Cation with N-Donor Bases
A. N. Toraman, L. Fischer, A. Pérez-Bitrián,* A. Wiesner, K. F. Hoffmann, S. Riedel*
Chem. Commun. 2024, 60, 1711–1714.
A. Pérez-Bitrián,* J. Munárriz, J. S. Sturm, D. Wegener, K. B. Krause, A. Wiesner, C. Limberg, S. Riedel*
Inorg. Chem. 2023, 62, 12947–12953.
17. Reactivity of [AuF3(SIMes)]: Pathway to Unprecedented Structural Motifs
M. Winter, M. A. Ellwanger, N. Limberg, A. Pérez-Bitrián, P. Voßnacker, S. Steinhauer, S. Riedel*
Chem. Eur. J. 2023, 29, e202301684.
D. Ardura,* Á. Zamora, A. Pérez-Bitrián
Chem. Educ. Res. Pract. 2023, 24, 1174–1189.
15. Gold Teflates Revisited: from the Lewis Superacid [Au(OTeF5)3] to the Anion [Au(OTeF5)4]−
M. Winter, N. Peshkur, M. A. Ellwanger, A. Pérez-Bitrián, P. Voßnacker, S. Steinhauer, S. Riedel*
Chem. Eur. J. 2023, 29, e202203634.
14. Terminal Au–N and Au–O Units in Organometallic Frames
A. Pérez-Bitrián, S. Alvarez,* M. Baya, J. Echeverría, A. Martín, J. Orduna, B. Menjón*
Chem. Eur. J. 2023, 29, e202203181.
13. Air-stable aryl derivatives of pentafluoroorthotellurate
D. Wegener, K. F. Hoffmann, A. Pérez-Bitrián, I. Bayindir, A. N. Hadi, A. Wiesner, S. Riedel*
Chem. Commun. 2022, 58, 9694–9697.
12. Unravelling the Role of the Pentafluoroorthotellurate Group as a Ligand in Nickel Chemistry
A. Pérez-Bitrián, K. F. Hoffmann, K. B. Krause, G. Thiele, C. Limberg, S. Riedel*
Chem. Eur. J. 2022, 28, e202202016 (HOT PAPER).
11. Hydrogen bonding to metals as a probe for an inverted ligand field
A. Pérez-Bitrián, M. Baya, J. M. Casas, A. Martín, B. Menjón*
Dalton Trans. 2021, 50, 5465–5472.
D. Ardura,* Á. Zamora, A. Pérez-Bitrián
Chem. Educ. Res. Pract. 2021, 22, 43–61.
M. Winter, N. Limberg, M. A. Ellwanger, A. Pérez-Bitrián, K. Sonnenberg, S. Steinhauer, S. Riedel*
Chem. Eur. J. 2020, 26, 16089–16097 (HOT PAPER).
8. C−N Cross-Couplings for Site-Selective Late-Stage Diversification via Aryl Sulfonium Salts
P. S. Engl, A. P. Häring, F. Berger, G. Berger, A. Pérez-Bitrián, T. Ritter*
J. Am. Chem. Soc. 2019, 141, 13346–13351.
D. Ardura,* A. Pérez-Bitrián
Chem. Educ. Res. Pract. 2019, 20, 618–632.
D. Ardura,* A. Pérez-Bitrián
Chem. Educ. Res. Pract. 2018, 19, 905–918.
5. An Organogold(III) Difluoride with trans Arrangement
A. Pérez-Bitrián, M. Baya, J. M. Casas, A. Martín, B. Menjón,* J. Orduna
Angew. Chem. Int. Ed. 2018, 57, 6517–6521.
4. Gold(II) Trihalide Complexes from Organogold(III) Precursors
M. Baya, A. Pérez-Bitrián, S. Martínez-Salvador, A. Martín, J. M. Casas, B. Menjón,* J. Orduna
Chem. Eur. J. 2018, 24, 1514–1517 (HOT PAPER).
3. (CF3)3Au as a Highly Acidic Organogold(III) Fragment
A. Pérez-Bitrián, M. Baya, J. M. Casas, L. R. Falvello, A. Martín, B. Menjón*
Chem. Eur. J. 2017, 23, 14918–14930 (HOT PAPER).
2. Anionic Derivatives of Perfluorinated Trimethylgold
A. Pérez-Bitrián, S. Martínez-Salvador, M. Baya, J. M. Casas, A. Martín, B. Menjón,* J. Orduna
Chem. Eur. J. 2017, 23, 6919–6929.
1. Gold(I) Fluorohalides: Theory and Experiment
M. Baya, A. Pérez-Bitrián, S. Martínez-Salvador, J. M. Casas, B. Menjón,* J. Orduna
Chem. Eur. J. 2017, 23, 1512–1515.
Team
Moritz Bubenik
Master Student
Edgar Weber
Research Internship Student
Group picture
April 2025
Alumni
Our values
News
Contact
Pérez-Bitrián Lab
Humboldt-Universität zu Berlin Institut für Chemie Brook-Taylor-Straße 2 12489 Berlin Room 0’102 E-Mail: alberto.perez-bitrian@hu-berlin.de Twitter: @AlbertPebi Tel.: 030/2093-82777